Continuing from last month's blog post, we’ll look more at the research of Dr. Ian McGilvray on the therapeutic potential of nanoparticles for treating pathologies of the liver. Assistant Scientist Dr. Sonya MacParland generously provided an in-depth perspective.
EXPLORING PHENOTYPE & NANOPARTICLE UPTAKE
Following the Nature Materials publication, the team took a closer look at how different immune cell populations interact with nanoparticles differently. The cells they wanted to investigate specifically were the M1 and M2 liver macrophages (Kupffer cells), and their subtypes.
When a macrophage expresses an M1 or M2 phenotype, it is said to be polarized. M1 macrophages encourage inflammation, M2 macrophages decrease inflammation and encourage tissue repair and immune regulation. The macrophages of the liver are a very heterogeneous population. They can be M1 or M2 based on what’s happening in the liver, most notably the presence or absence of inflammation.
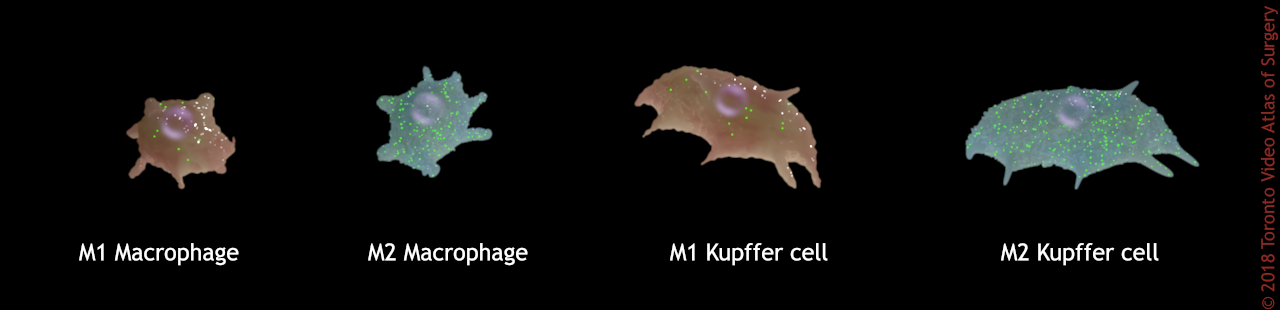
M1 macrophages encourage inflammation, M2 decrease inflammation and encourage tissue repair.
Does a cell’s phenotype/polarization affect its likelihood to uptake nanoparticles? In a new paper published in ACS Nano, the team showed how if you take a liver macrophage bulk population, and separate them into M1 vs M2 based on surface markers, you will see more uptake of nanoparticles in M2, immunoregulatory macrophages.
If M2 macrophages are taking up more nanoparticles, this implies that in an inflammatory environment, where conditions increase the numbers of M1 macrophages, fewer nanoparticles will be trapped by liver macrophages, and can bypass the liver and have a higher chance of reaching a tumour elsewhere in the body.
Abstract
A significant challenge to delivering therapeutic doses of nanoparticles to targeted disease sites is the fact that most nanoparticles become trapped in the liver. Liver-resident macrophages, or Kupffer cells, are key cells in the hepatic sequestration of nanoparticles. However, the precise role that the macrophage phenotype plays in nanoparticle uptake is unknown. Here, we show that the human macrophage phenotype modulates hard nanoparticle uptake. Using gold nanoparticles, we examined uptake by human monocyte-derived macrophages that had been driven to a “regulatory” M2 phenotype or an “inflammatory” M1 phenotype and found that M2-type macrophages preferentially take up nanoparticles, with a clear hierarchy among the subtypes (M2c > M2 > M2a > M2b > M1). We also found that stimuli such as LPS/IFN-γ rather than with more “regulatory” stimuli such as TGF-β/IL-10 reduce per cell macrophage nanoparticle uptake by an average of 40%. Primary human Kupffer cells were found to display heterogeneous expression of M1 and M2 markers, and Kupffer cells expressing higher levels of M2 markers (CD163) take up significantly more nanoparticles than Kupffer cells expressing lower levels of surface CD163. Our results demonstrate that hepatic inflammatory microenviron- ments should be considered when studying liver sequestration of nanoparticles, and that modifying the hepatic microenvironment might offer a tool for enhancing or decreasing this sequestration. Our findings also suggest that models examining the nanoparticle/macrophage interaction should include studies with primary tissue macrophages.
Basically, M1 macrophages are anxious, panicky freak-outs. They’re berzerkers. They’re too distracted with fueling a chaotic inflammation riot to notice all these foreign particles whizzing by. M2 macrophages are calm stewards of the body’s temple. They meticulously clean and repair their surroundings, and swat every fly that cruises through their section of the garden. They don’t miss a thing.
Another relevant form of the macrophage in the liver-cancer scenario are the tumour-associated macrophages or TAMs, which are macrophages taken over by, and working for, cancer (also referred to as tumour-infiltrating macrophages or TIMs). Think of these as demonically-possessed, zombie macrophages.
Cancer patients with higher numbers of TAMs present in their tumours will likely have a worse prognosis. TAMs closely resemble the M2, immunoregulatory phenotype, and promote rapid increase in cancer cells, the generation of new blood vessels that will feed tumours, and remodel the neighbouring cellular microenvironment to the cancer’s benefit. So insidious!
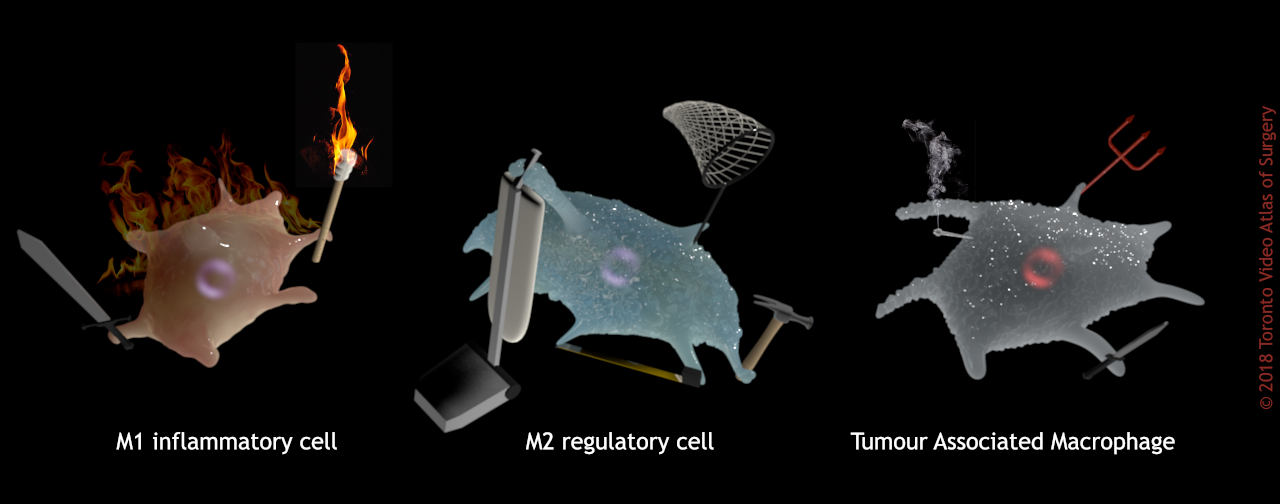
Macrophage phenotype (function) influences their proclivity for sequestering nanoparticles.
Understanding the patterns of uptake and sequestration of nanoparticles by different macrophage subtypes may be critical to explaining how nanoparticle size, shape, and surface characteristics affects how they interact with immune cells. This can also help us understand how nanoparticles affect cellular and organ function.
One cellular function worth investigating is an immune cell’s ability to change forms. Does uptake of nanoparticles affect an immune cell’s ability to specialize? Monocytes originate in the bone marrow before migrating through the bloodstream to an organ in the body where they take up residence and become a tissue-specific macrophage. Freshly isolated monocytes were exposed to nanoparticles, and then exposed to conditions that would cause them to polarize into macrophages. It was found that monocyte uptake of nanoparticles prior to specialization did not prevent the monocytes from transforming into mature macrophages. Whew!
Although roughly the same amount of cells from various subtypes took up nanoparticles, they did so with various degrees of enthusiasm. There was significantly higher nanoparticle uptake for all macrophage types compared to monocytes, and a significant difference in the amount eaten for M1 macrophages compared to M2 macrophages. The data provides evidence that nanoparticle uptake by macrophages can be modified by inflammatory states: polarizing macrophages with inflammatory stimuli rather than with more “regulatory” stimuli reduces per cell nanoparticle uptake by an average of 40%. Also observed, was that there is a point at which the macrophages become saturated with nanoparticles, and stop picking up more.
What about the long-term effects?
Do nanoparticles harm or impair macrophages? Nanoparticle treatment, even for extended periods of time, appears to have no impact on macrophage viability or phagocytic ability but does reduce the proinflammatory potential of macrophages. Nanoparticle uptake significantly impeded the ability of monocyte-derived macrophages to produce inflammatory cytokines, most especially in the subtypes that take up more nanoparticles. These findings show that certain aspects of macrophage inflammatory function, such as cytokine secretion, are altered by nanoparticle uptake, but macrophage cell death and phagocytosis are not impacted, suggesting that this is a selective, non-toxic effect.
Primary human liver macrophages are far better than circulating monocytes at nanoparticle ingestion, but even so, polarizing them with M1-skewing cytokines significantly inhibits nanoparticle ingestion in these otherwise nanoparticle-avid cells. Results demonstrate that nanoparticles are preferentially taken up by monocyte-derived macrophages and Kupffer cells that have an M2-like phenotype. The uptake of nanoparticles impacts the ability of these macrophages to produce proinflammatory cytokines, but does not alter the cell’s ability to work successfully or its phagocytic ability.
If a tiny disturbance is created in the liver that will induce temporary inflammation, it appears we help more nanoparticles to be taken up in a target site. Modification of the liver’s cellular environment will play an important role in future applications of nanoparticle treatments.
FUTURE DIRECTIONS
The next study, already underway, will focus on tumours, especially the insidious TAMs. Currently, we’ve just been using nanoparticles to track, but how can we target TAMs using gold nanoparticles to deliver therapeutics? TAMs surround and are found within tumours. They protect the cancer by secreting cytokines that turn away T-cells, preventing them from getting a close look and killing the tumour.
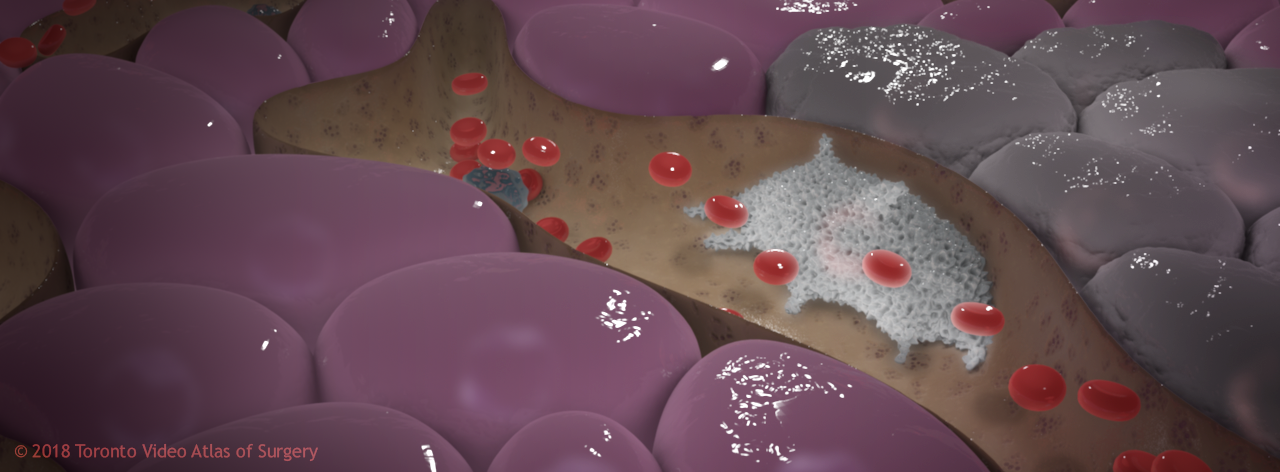
TAM protecting cancerous liver cells
Another challenge to liver tumours is that they can be very small and very diffuse, which makes sense based on the blood flow dynamics of the liver; blood slows down within the liver and is slowly filtered through. If tumours are small and diffuse, they can’t be resected surgically. If we deliver a chemotherapeutic to the liver (via transarterial chemoembolization or TACE), often the tumours will grow back because it’s hard to target them.
The best solution may be to take a two-pronged approach: target the cancer with a chemotherapeutic, and use immunomodulatory nanoparticles as a “cleanup crew”. This will create a strong anti-tumour environment in the liver, helping to get rid of the TAMs that protect the tumour and deliver a cytokine signal that helps activate T-cell immunity.
At any given time there are many cells in the human body that have the potential to become tumours. Our bodies are constantly recognizing them, flagging them and killing them. So the idea would be to take advantage of this by deleting the macrophages that are preventing this chain reaction from taking place. The approach would be to either delete the macrophages by targeting them with chemotherapeutic nanoparticles, or, change the phenotype of these macrophages by targeting them with a cytokine nanoparticle. We could then take advantage of these altered macrophages to stimulate an anti-tumour immunity.
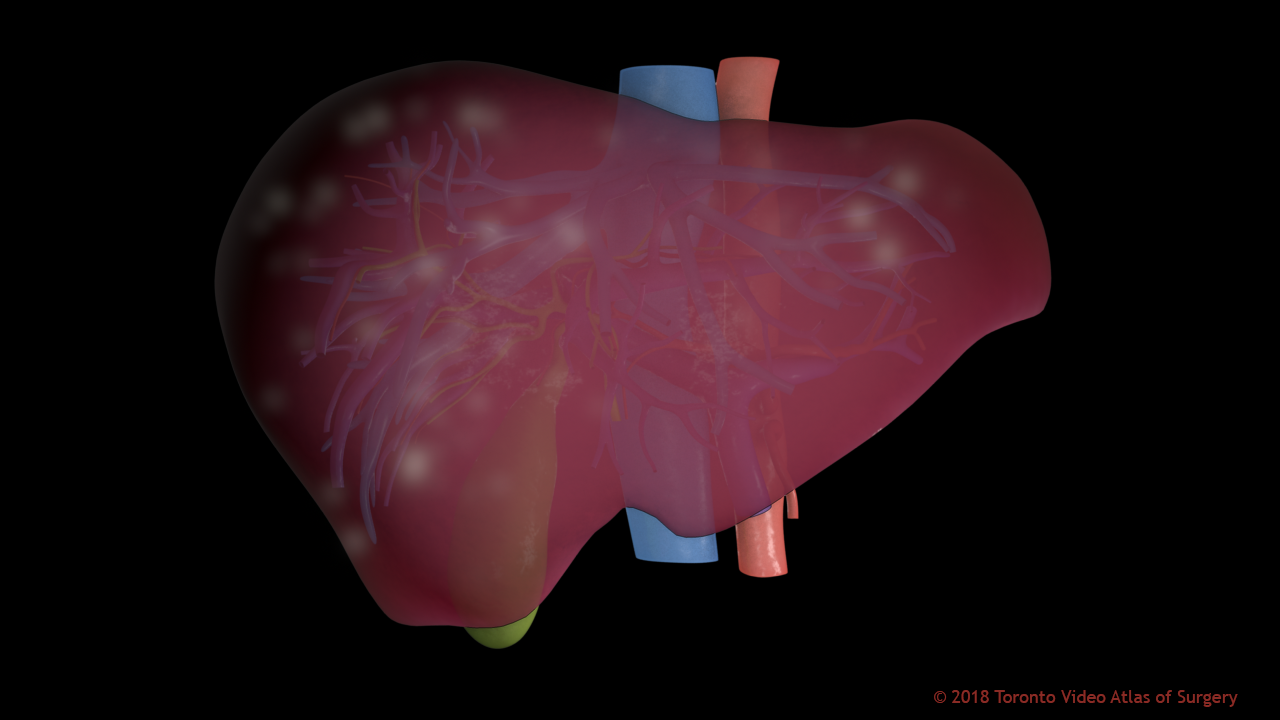
Tumours in the liver can appear anywhere, and may be too small & numerous to remove surgically
In order to show that you can therapeutically target tumours, you need a really good tumour model, but the problem with tumour models is that so many of them are induced. To study tumours, typically tumour cell lines are injected into animals with a preselected “good” tumour cell. Do these ideal tumour cells really need an altered immune environment to grow? Maybe not.
In a collaborative study set up with Dr. Thomas Michalak at the Memorial University of Newfoundland, woodchucks are infected with woodchuck-specific hepatitis virus, which has almost identical genome to human hepatitis B virus. Most human liver tumours are result of either hepatitis B or hepatitis C infections–oncogenic (cancer-producing) viruses. The resulting tumours in the animals’ livers are not induced, they are spontaneous. By injecting the virus into these livers, the environment of these animals’ livers are altered so that cells will transform into cancer; tumours will develop. But these tumours develop in an intact immunological background.
The next step will be to look at nanoparticle uptake in human tissue samples. Tools are being developed for the animal study by adapting existing human study tools. They will then be able to inject the woodchucks with nanoparticles and look at whether the tumours regress, stay the same, or grow. Then they will measure which immunological markers are responsible for that tumour growth, stabilization, or regression.
Dr. McGilvray works with a multitude of partners at the University of Toronto on transdisciplinary studies looking at liver cancers and organ transplantation. He was recently appointed the director of research for Multi-organ Transplant at UHN. His future research directions, in addition to the studies on nanomedicine, will include addressing the problems with induced vs. spontaneous tumour models, focuses on specific cell populations within the liver, and how to create anti-tumour environments in the liver. By defining a normal human liver, and a diseased human liver, we can then look at how each of these cell populations interact with nanoparticles, how to target specific cells in the liver, and how to direct nanoparticles past the liver to target other tissues of the body.
Dr. MacParland has recently started her own lab for nanoparticle studies, and will also focus on immunological tools and readouts. Her research will investigate immunologically what nanoparticle targeting does, and how to make it effective, what’s happening immunologically when nanoparticles are taken up, and how that can be leveraged to enhance either the efficacy of the treatments, or enhance targeting to a specific location.
Dr. MacParland is also an active member of CanHepC, a network looking at different aspects of Hepatitis C infection. This is a Canada-wide program that trains people to be clinicians or scientists that work in all aspects of disease treatment, dissemination of information, enhancing uptake of treatment, modelling cost-effectiveness of treatment, and modelling response to therapy. CanHepC will be meeting in Toronto in February of 2018.
Thanks for taking the time to learn about the nanoparticle research being done at the McGilvray & MacParland research labs! If you'd like to learn more, you can reach Dr. Ian McGilvray at Ian.McGilvray@uhn.ca and Dr. Sonya MacParland at Sonya.MacParland@uhnresearch.ca
